So, I was messing around with a balloon the other day, you know, just one of those cheap party ones. I grabbed it and started blowing it up. It’s funny how you do these things without really thinking, right?
Anyway, as I was huffing and puffing, watching this flimsy piece of rubber get bigger and bigger, I actually stopped and thought: what’s really going on here? How does my breath, just air, make this balloon inflate and get all tight?
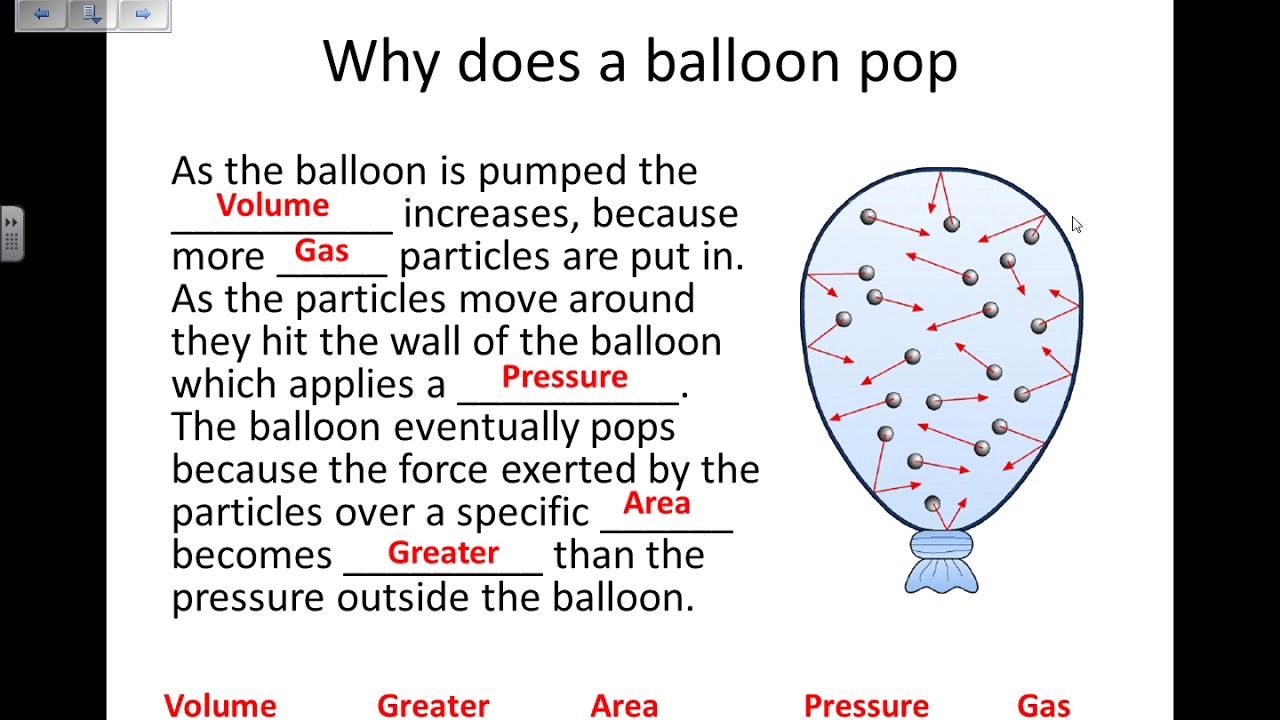
I started picturing the air I was blowing in. It’s not just empty space, obviously. Air is made up of gas, tons and tons of tiny, invisible particles. Like, super tiny. And these little guys, they’re not just chilling in there. Once they’re inside the balloon, they’re zipping around like crazy, bouncing off each other, and, crucially, bouncing off the inside walls of the balloon.
I kept blowing, and the balloon got firmer. I could feel it pushing back against my fingers. That’s the pressure, isn’t it? Each one of those little gas particles, when it smacks into the rubber, gives it a tiny push. Now, one tiny push from one tiny particle doesn’t do much. But there are billions and billions of them in there, all smacking the inside of the balloon, all the time, from every direction. It’s like a constant bombardment.
That’s the pressure! It’s just the grand total of all those tiny pushes from all those gas particles hitting the balloon from the inside.
So, I thought, what happens if I blow more air in?
- I blew some more.
- The balloon got bigger, and it got much tighter.
More air means more gas particles. More particles mean more collisions with the balloon wall per second. So, more pushes, and that means higher pressure. Simple as that, really, when you break it down.
The balloon itself, the rubber, it’s stretchy. It tries to hold its shape, or at least shrink back. So there’s this battle: the gas particles pushing outwards, and the stretchy balloon material trying to pull inwards. As long as the outward push from the gas is stronger, the balloon stays inflated.
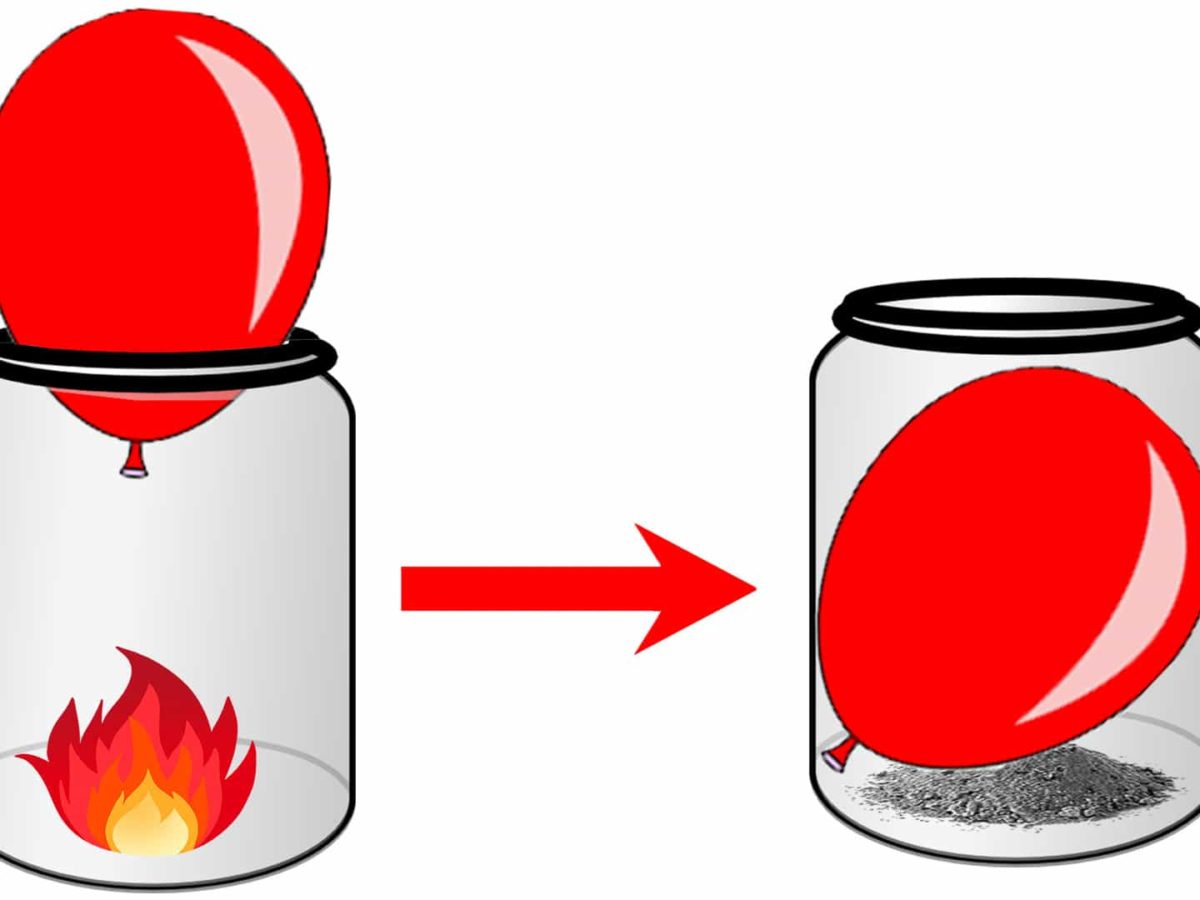
Then I got to thinking, what if I push it too far? I kept blowing, feeling a bit mischievous. The balloon got super taut. You could tell those gas particles were really packed in there, hammering away at the walls. And then, of course, POP! Scared the cat. But it made sense. The pressure from all those energetic gas particles just became too much for the rubber to handle. The material failed because the force from the inside was just too great.
I grabbed another balloon, blew it up, but not too much this time. Then I just let the opening go. Whoosh! The air rushed out, and the balloon zipped around the room. Why did the air rush out? Well, all those particles inside were under pressure, all squeezed together. The air outside the balloon? Much lower pressure, more space for them. So, the moment they had a chance, they rushed from the crowded, high-pressure area inside to the less crowded, low-pressure area outside. They just want to spread out.
My Little Summary of the Whole Thing
So, after my little experiment and a bit of thinking, that’s how I see it. Gases create pressure in a balloon because you’re essentially trapping a whole bunch of hyperactive, tiny particles (the gas) inside an elastic container (the balloon). These particles are constantly moving and smashing into the inner surface of the balloon. Each collision is a tiny force, but with zillions of them happening every second, it adds up to a significant outward push. That push is what we call pressure, and it’s what keeps the balloon inflated and feeling firm. More gas, more collisions, more pressure. Until, well, you know what happens if there’s too much!
It’s pretty straightforward when you stop and visualize those little guys doing their dance. No fancy science degree needed to get the gist of it, just a balloon and a bit of curiosity, I guess.
